|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
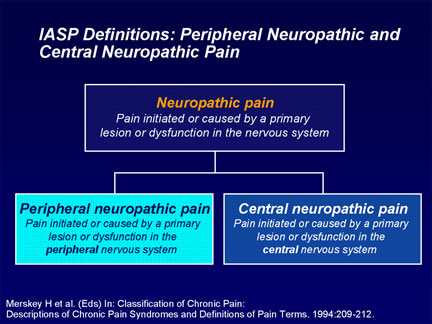
J. Robin Conway M.D. Abstract Diabetic Peripheral Neuropathy (DPN) is among the most common and troublesome complications of Diabetes resulting in reduced quality of life, increased morbidity and mortality as well as increasing costs of treatment. Since in common with other microvascular complications, the development and progression of neuropathy tends to be glucose dependant; adequate glycemic control should almost eliminate this devastating complication. Factors in the pathogenesis of neuropathy are altered metabolism, vascular insufficiency, loss of growth factor trophism, and autoimmune destruction of nerves in a visceral and cutaneous distribution. The diagnosis and pathophysiology of DPN is discussed leading to rational prevention and treatment. The pharmacotherapy of DPN is reviewed. Definition Diabetic neuropathy is a heterogeneous disorder that encompasses a wide range of abnormalities affecting both proximal and distal peripheral sensory and motor nerves, as well as the autonomic nervous system (ANS). Neuropathy affects 30-60% of Diabetics and 10-20% have Neuropathic Pain which is defined as.”An unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”. Pain is either acute or chronic. Acute pain is usually recent, of less than a months duration and related to tissue damage. It serves a protective function and resolves as healing takes place. Chronic pain has usually lasted more than 6 months, persists even after healing, has no protective function and adversely effects quality of life. Pain may be either Nociceptive (due to local trauma & inflammation) or Neuropathic. Neuropathic pain is defined as, “Pain initiated by a defect or malfunction of the Nervous System”. Peripheral pain is that caused by a lesion of dysfunction in the peripheral nervous system (such as post-herpetic pain), Central Pain is caused by a lesion of the Central Nervous System (such as a stroke). Some pain (such as pain of lumbar arthritis with radiculopathy) may have both nociceptive and neuropathic components. Neuropathy is frequently bilateral and may present as stocking & glove dysthesia. There are 2 components to the pain; the afferent stimulus from the peripheral nerves and the influence of the central nervous system. Vocabulary
In neuropathic pain abnormal sensations may be transmitted along Aß , Ax or C fibers
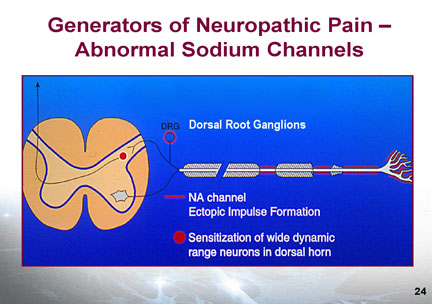
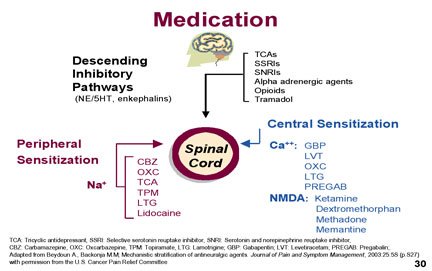
Diagnosis Neuropathic pain may be either spontaneous as a continuous or intermittent burning, stabbing, shooting or electrical shock sensation. The pain may be evoked or worsened by touch or cold contact (allodynia) and may be accompanied by paresthesia or dysthesias. There may be associated sensory abnormalities such as loss of fine (10 gm) touch, loss of temp sense or vibration sense (256 cps tuning fork). There may also be autonomic changes manifested by colour or temperature changes. The most common presentation is a continuous numbing pain (stocking & glove anesthesia) with superimposed paroxysms of tingling, burning or shooting pain. The examination should include sensory & motor exam of the painful area and fine touch testing with a 10 gm Semmes-Weinstein Filament, a pin, cotton or a fine brush, a 256 cps tuning fork and a source of heat and cold (special rollers or water filled test tubes. Findings may include hypoalgesia or hyperalgesia, allodynia, abnormal latency or spatial changes. Autonomic signs of vasoconstriction or vasodilation may be present. A score of >4/10 in the DN4 questionnaire indicates neuropathic pain. Pathophysiology In normal nociceptive pain, trauma triggers nociceptive receptors which generate an action potential in the peripheral nerve. Afferent stimuli from the peripheral nerves are transmitted to the dorsal horn of the spinal cord which processes and transfers peripheral stimuli up the spino-thalamic tract to the brain where the signal is interpreted as sensory and affective components in the sensory cortex and pre-frontal (affective) cortex. Mono-amine pathways in the brain and central nervous system modulate the processing, transmission and perception of pain signals. The amplitude of the transmitted pain signal from the dorsal horn to the brain is determined not only by the excitatory input but also by the inhibitory mono-amine impulses descending from the brain. Mechanisms of Neuropathic pain may include peripheral sensitization with spontaneous peripheral nerve activity mediated by sodium channels. The cause of this may be local tissue injury or ischaemia causing the release of multiple Inflammatory factors including substance P, Interleukin 1b, bradykinins, nerve growth factor histamine etc. These cause damage to the peripheral nerve with increase in sodium channels leading to hyperexcitability of the nerve. Medications that target sodium channels such as carbamazepine, lidocaine, topiramate and Tricyclics may therefore modify this pain of peripheral sensitization.
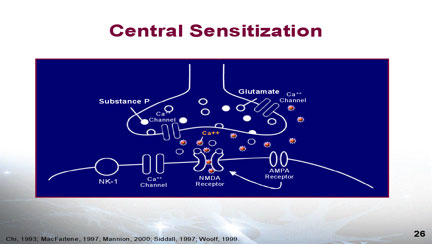
Central sensitization in the dorsal horn of the spinal cord or the Central Nervous System involves Calcium Channels and AMPA and NMDA receptors. The release of excitary neurotransmitters and neurotramsmitters including glutamate & substance P may produce central sensitization of the neurons of the dorsal horn. The central sensitization is mediated by the calcium channels and the increased calcium transport causes spontaneous impulses (action potentials) conveying a pain message to the brain. The activity of the calcium channels may be decreased with the anticonvulsant drugs gabapentin , pregabalin or lomotrigine. NMDA receptors also play a role in central sensitization and NMDA antagomists such as methadone, dextromethorphan, ketamine & amantidine will decrease the nerve impulses. Allodynia is an indication that central sensitization has occurred in the dorsal horn.
Centrally Mediated Pain Inhibition Although it is likely that many mechanisms play a role in pain relief, one of the commonly accepted theories involves the role of both serotonin and norepinephrine. In the spinal cord, these neurotransmitters exert an analgesic effect in descending pain pathways and are part of the body's endogenous analgesic system. Increased activity of the serotonergic and noradrenergic pathways have an inhibitory effect on pain transmission. Non-Pharmacologic Treatment Thermal: hot or cold compresses Other Interventions Injections: trigger points, nerve block, epidural or intrathecal injection Pharmacologic Treatment There may be multiple factors at play with neuropathic pain including Peripheral Sensitization, which may respond best to drug that decreases sodium channel activity such as carbamazepine, tricyclics, topiramate or lidocaine; or Central Sensitization (if allodynia present) which may respond to a drug that decreases calcium channel activity such as gabapentin, pregabalin, lamotrigine. To block NMDA receptors, use dextromethorphan, methadone, ketamine or memantine. To stimulate descending inhibitory pathways use tricyclics, SNRI (more effective than SSRI), alpha adrenergic agents, opioids, tramadol. If Monotherapy does not give adequate relief us different medications with different mechanisms of action. Only about half of individuals get an adequate (30% improvement) on one drug. Logical drug combinations will target different mechanisms of action. We normally use agents from different classes.
Adjuvant Therapy Acetaminophen and NSAIDS on their own are not normally very effective for neuropathic pain but may be beneficial when combined with other agents. Similarly 10% Lidocaine cream may help with other agents particularly where there is peripheral sensitization. Diclofenac liquid (Pennsaid) may also be helpful as is 10% Ketamine in Diffusamax. Small doses of Ketamine orally, dilute 10 mg/ml injectable with 9cc water to make 1 mg/ml soln & start with 1 ml. Opioids Opioids bind to receptors on specific neurons in the central and peripheral nervous system, resulting in suppression of neuronal firing. The main opioid receptors are the mu receptors which are associated with analgesia but also with respiratory depression, euphoria and addictive potential. The kappa receptors, which mediate analgesia, miosis and sedation but can also cause dysphoria. The delta receptors which are associated with analgesia and changes in affect. Oxycodone which is primarily a mu agonist and also has mild kappa agonist properties has been shown to have benefit in post-herpetic and diabetic neuropathy. Codeine is a mu agonist, it has to be metalolized in the body to morphine and 10% of the population lack the enzyme to do this so they get no analgesia although they get the side effects of drowsiness, constipation etc. 60 mg=10 mg morphine. Long acting Codeine Contin is also available. Meperidine (Demerol) is a mu agonist with NMDA blocking properties. It is short acting, 30 mg meperidine= 10 mg morphine. Not recommended for chronic use due to accumulation of a toxic metabolite. Morphine: mu agonist. Metabolized to morphine-3-glucuronide, high levels of this may interfere with analgesia and cause hyperalgesia. Available in liquid, tablets and extended release MS-Contin. Fentanyl: Mu receptor agonist, 70 times as potent as morphine 25 mcg=10 mg morphine. Available as a transdermal patch (25, 50 or 100 mcg/hr) which need to be changed every 72 hrs. Methadone: Mu & delta agonist that also blocks the NMDA receptor (important in neuropathic pain. 1 mg=10 mg morphine. Long half life. Prescriber must have a methadone license. Tolerance to opioids may develop with time. Opioids work best when dosed to effect, SR preparations may be helpful. The use of these drugs is often a balancing act between analgesic effect and side effects. Addictive potential must be considered. With development of tolerance, switching to another agent in equianalgesic dose may help. Tramadol: (Tramacet 37.5 mg tramadol with 325 mg acetaminophen). Non narcotic mu receptor agonist 2 tabs equianalgesic with 60 mg codeine. Dose is 2 tabs q4h prn (maximum 8/day). Autonomic Neuropathy Impaired function of small unmyelinated fibres of the autonomic nervous system. Affects 40% of diabetics but symptoms are seen in less. Associated with peripheral neuropathy and nephropathy. Symptoms include Erectile dysfunction, anhidrosis (with dry feet & legs), overflow incontinence, abdominal fullness & nausea (due to gastroparesis), gustatory sweating, postural dizziness. Signs include dry skin particularly over the lower half of the body, tachycardia with no sinus arrythmia, postural hypotension, paradoxical picture of highest BP overnight and lowest in AM (non dipper). Diagnosed by a >20 mm drop in systolic BP on standing. Because diabetics with autonomic neuropathy are unable to adapt rapidly to changes in intravascular volume or postural changes, dehydration should be avoided as well as rapid postural changes. Anticholinergics may help gustatory sweating (but may worsen urinary retention). The nausea of gastroparesis may respond to small meals or mealtime doses of metoclopramide or domperidone. References:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||