Epidemiology of Ulceration and Amputation
Approximately 15% of persons with diabetes will have an ulcer in their lifetime,[15,16] and 0.5% to 29.0% will have neuropathic joint changes.[17,18] Diabetes is the leading cause of nontraumatic amputations, amounting to 57,000 per year or 150 per day. One half to 80% of all amputations are diabetes-related.[16,19-21] It is postulated that 50% of these can be prevented through a comprehensive lower extremity amputation (LEA) prevention program,[22] which is part of the goals for Healthy People 2010 (Table 2).[23]
The cost of foot disease is astounding. Medicare records show that $1.5 billion was spent directly on diabetic foot ulcers from 1995 to 1996.[24] Almost three fourths of this was spent during inpatient treatment alone. Today, the annual cost of diabetic foot ulcer care is $5 billion in direct cost and $400 million in indirect cost.[25] Unfortunately, 70% of people with ulcers have little or no regular follow-up care, which is necessary to prevent progression.[25] In a study of inpatient ulcer care, only 1.6% had prescriptions for off-loading materials at discharge, and only 11.0% had arrangements for home health wound care.[26]
The sequelae of ulceration, including amputation, cost of prosthesis, and rehabilitation after amputation is enormous. The direct cost of LEA ranges from $20,000 to $60,000 per patient.[22,27-31] In 1992, the cost of rehabilitation was $14,500 to $21,500 per patient.[32] This does not include the cost of prosthesis.
Morbidity and Mortality
Among the patients having an estimated 57,000 to 125,000 LEAs per year,[33-35] 5% to 17% will die during the operation and 2% to 23% will die within 30 days of surgery.[36,37] Ipsilateral reamputation will be required in 8% to 22% of the survivors, and 26% to 44% will require a contralateral amputation within 4 years. Five-year survival is 40% overall, but only 25% in the very elderly (>80 years).[38] Despite surgical advances, these rates remain staggering.
In addition to the mortality of LEA, the morbidity is also considerable. After below-knee amputation, the work of walking increases, and amputees decrease their walking speed to maintain their rate of oxygen uptake (measured in milliliters per kilogram per minute). Above-knee amputees will have both decreased walking speed and increased rate of oxygen uptake.[39,40] A year-long study of amputees in a Texas hospital found that of the 97.3% admitted for amputation from home, 18.5% were discharged to a nursing home and 7.0% to a rehabilitation facility.[41] Not a single patient improved from their baseline functional activity. The extra weight-bearing load placed on the remaining extremity increases the contralateral risk for ulceration. This may explain why compliance with prosthesis declines over time and as few as 5% of amputees can walk safely outside of their home on uneven surfaces.[42,43]
Pathogenesis
Distal symmetric peripheral neuropathy affects up to 50% of diabetics within 15 years after diagnosis.[16] The etiology of this nerve damage is not well understood. The polylol theory postulates that biomechanical reactions related to hyperglycemia reduce blood glucose to sorbitol, which is thought to be toxic to tissues.[44,45] Another theory proposes that hyperglycemia damages the blood vessels supplying nerves and impairs neurotransmission.[15] Sensory neuropathy can be defined as loss of sensation as measured by the Semmes Weinstein 10 g monofilament, 4-question verbal neuropathy score, or vibration perception threshhold test[46] and is a major risk factor for ulceration. The ADA recommends the use of a 10 gram nylon Semmes Weinstein monofilament as an accurate and inexpensive way to evaluate sensory loss.[10,12,25,32,41,46,47] Disposable filaments and methods to screen and manage diabetic foot neuropathy can be obtained from the Lower Extremity Amputation Prevention (LEAP) Program of the Bureau of Primary Health Care (BPHC).*
Distal muscle atrophy is also common. Loss of motor nerve function causes weakening of the intrinsic foot muscles. This imbalance produces changes in foot structure and gait. The resulting deformity and limited range of motion contribute to increased mechanical stress on corresponding areas of the foot. Toe deformities can be easily recognized by the medical professional. Extension contracture at the metatarsophalangeal (MTP) joint with flexion contracture at the proximal interphalangeal (PIP) joint is commonly referred to as a hammer toe while hyperextension of the MTP and flexion of the PIP and distal interphalangeal (DIP) joint is termed a claw toe. Claw and hammer toes are a sign of distal muscle atrophy and neuropathy.[48] Claw toes increase pressure on the metatarsal heads and dorsal interphalangeal joints. Hammer toes can result in pressure at the distal ends of the toes. Pressure may lead to callus formation and ulceration.[49] Hallux rigidus, or limitation of dorsiflexion of the great toe, also predisposes to ulceration,[50] since the toe-off phase of gait requires 45° of metatarsophalangeal joint extension.[51]
* Bureau of Primary Health Care, Division of Programs for Special Populations, 4350 East West Highway, 9th Floor, Bethesda, MD 20814. Telephone: 1-888-275-4772.
Prevention
The best and most cost-effective way of preventing diabetic foot disease and amputation is to interrupt the pathway to amputation by preventing ulcer formation.[52]
Foot Screening
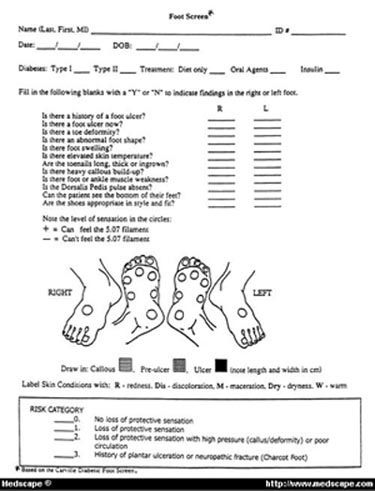
An annual foot screen by a health care provider is recommended for all diabetic patients. The foot screen presented in this paper was developed in the Carville and LSU Diabetic Foot Clinics (Figure). Health care professionals can easily learn how to identify a "high-risk" foot quickly and cost effectively.
Recurrence rate of an ulcer is 70%.[53] During the final stage of wound healing, scar tissue forms and there is a progressive increase in tissue strength due to cross-linking of collagen fibers along tension lines. A healed wound will gain 20% of its strength within 1 week and 60% in 4 to 6 weeks and will plateau somewhere between 70% and 90% over a period of 2 years.[54] The area of ulceration therefore will never be "normal" again and will be more vulnerable to injury and reulceration. The patient will automatically be placed into the highest risk category and should be referred to the appropriate wound and/or foot specialty clinic or supplier for protective footwear (Table 3).[55-57] The Medicare Therapeutic Shoe Bill pays for a portion of these services.
Toenails
Long, thick, or ingrown toenails can produce ulceration or infection. Regular and proper nail care is an important preventive measure in managing a patient with lower extremity neuropathy. Patient education regarding safe and effective nail care methods is critical. They should trim their nails parallel with the distal surface of the toe, and seek assistance from a professional if the nails are too thick to safely trim themselves.
Calluses
Localized callus formation on the plantar surface of the foot indicates an area of high mechanical stress and is a risk factor for ulceration.[58] Formation of an ulcer beneath a callus has been well documented. Discoloration beneath a callus or hemmorhage on the lateral border of a callus requires immediate debridement to prevent further complications.[59] Regular removal of calluses is an effective way to reduce pressure, thus decreasing the risk of ulceration.[60]
Protective Footwear
Appropriate footwear is integral to preventing ulcers.[61-64] Prescription footwear and custom fitted orthotics have been shown to prevent occurrence and recurrence of complications and increase patients' use of shoes outdoors.[61-65] One study compared a group of patients with history of ulceration or amputation wearing their own shoes versus patients who had custom footwear and orthotics. The group that wore their own shoes had more than double the rate of reulceration. However, this therapeutic modality is vastly underutilized. Findings from a random questionnaire sent to a group of eligible diabetics by the American Orthopedic Foot and Ankle Society suggested that only 12.2% wore prescription shoes and 15.4% wore custom foot orthoses.[66] Results of an effort to educate 43,000 Medicare beneficiaries with serious foot problems and their physicians about the Therapeutic Shoe Bill were disturbing.[67] Only 2% of the beneficiaries enrolled, and only 6% of the physicians notified enrolled any of their patients. Among the physicians who did enroll patients, only 26% were internists and 9% were family practitioners.[67] Another cohort of more than 60,000 Medicare beneficiaries showed that only 0.6% had therapeutic footwear.[68] The appropriate utilization of orthotics and footwear could greatly improve outcomes and decrease LEAs (Table 4).
Neuropathic Fracture
A neuropathic (Charcot) fracture is one resulting from chronic destruction of the bones and joints of the foot. The etiology of neuroarthropathy is poorly understood,[69,70] but it may involve a combination of sensorimotor neuropathy, minor trauma, autonomic neuroarthropathy with increased blood flow to the bone, corticosteroid-induced osteoporosis, and metabolic abnormalities that weaken the bone.[70]
Early recognition and management of an acute Charcot fracture is essential to minimize bone destruction. Charcot's arthropathy is diagnosed in 1% to 2% of the general diabetic population, while 13% to 29% of patients evaluated in specialty foot clinics are found to have Charcot joint changes.[17,18] This may be partly explained by referral bias but also likely represents underdiagnosis. Minor or unperceived trauma often precipitates the fracture.[71,72] Signs of fracture include redness, swelling, and more than 2°C skin temperature difference when compared with the contralateral foot. Dorsalis pedis pulses are often bounding.[73] The patient is afebrile unless a systemic infection is present. Elevation and rest of the extremity results in an immediate decrease in swelling. During the dissolution (inflammatory) phase, immobilization is required for a period of 3 to 9 months with total contact cast or healing boots. Non-weight-bearing is needed with modified gait and an assistive device.[18,69,71,72,74] Bisphosphonates may be of benefit.[75] The coalescence (healing) phase should show radiographic evidence of consolidation of bony fragments and should be treated with protected weight-bearing, a removable cast or a walker, ankle-foot orthosis, or pressure relief ankle-foot orthosis. At resolution (remodeling), therapeutic shoes and inserts are beneficial to prevent recurrence.[18,69,71,72,74] Surgery is indicated for unstable, malaligned, or nonreducible fractures.[69] Often confusing is the absence of pain. The majority of people who have a Charcot fracture have lost protective sensation, so that pain is no longer a reliable indicator. Osteomyelitis may occasionally be confused with a Charcot fracture but will usually have an overlying ulcer. However, a person with an ulcer can have a neuropathic fracture. Magnetic resonance imaging (MRI), radionucleotide bone scan, scintigraphy, or bone biopsy may be required to differentiate the two in difficult cases.[76,77]
Patient-Related Factors
Patients who have loss of vision, mobility, or flexibility may be impeded from doing a daily foot examination. A hand-held mirror placed on the floor or a wall mirror may assist patients with hip or knee problems. A family member may need to examine the feet daily if the patient has visual defects. Diabetics who live alone may be at a higher risk for ulceration because of the inability to perform daily self-care, though this has not been documented in the literature.
Conclusion
Multiple studies have documented the ability of preventive diabetic foot care to reduce complications and costs.[78-80] Patout et a[80] compared patient outcomes before and after 1 year of enrollment in a comprehensive LEAP program. The results showed a dramatic reduction in foot-related complications (Table 5). Armstrong and Harkless[78] have also shown that a multidisciplinary diabetes care team can result in fewer foot complications. Over a period of 3 years, their clinic had an average of only 1.1/1,000 amputations per year, compared with 11/1,000 amputations per year in the general diabetic population.[24] A model developed to estimate the expected incidence and cost of amputation showed that the economic benefits (discounted at 5%) using strategies of education, multidisciplinary care referral, and therapeutic shoes totaled $2 million to $3 million for a cohort of 10,000 diabetic patients.[81]
In summary, a strategy of yearly comprehensive foot examinations and education with appropriate interventions and risk reduction can be a cost-effective means of improving both the quality and duration of life in those with diabetes mellitus.
Table 1. Clinical Practice Recommendations and Standards of Care for Patients With Diabetes Mellitus
|
|
Test
|
Frequency
|
Target/Goals
|
| Hemoglobin A1c |
Every 3 months |
<7%
|
| Microalbumin |
Annually |
<30 mg/24 hour or 20 g/min timed collection
30 mg/L creatinine on random sample |
| Low-density lipoprotein |
Annually |
<100 mg/dL
|
| High-density lipoprotein |
Annually |
>45 mg/dL
|
| Triglyceride |
Annually |
<200 mg/dL (230 mmol/dL)
|
| Thorough foot examination |
At least annually
|
|
| Dilated eye examination |
Annually
|
|
| Exercise |
30 min of moderate activity |
Most days of the week |
Table 2. Goals of Healthy People 201023
|
| Target |
Lower extremity amputation (LEA) rate of 1.8/1,000 diabetics per year
|
| Baseline |
4.1/1,000
|
| Target setting |
55% improvement
|
Table 3. Indications for Referral to Comprehensive Diabetic Foot Care Program
|
- History of ulcer or current ulcer
- Loss of protective sensation
- Foot deformity
- New onset/diagnosis of diabetes mellitus
- Decreased joint mobility
- Retinopathy
- Heavy callus
- History of amputation
- History of neuropathic fracture
|
|
|
|
|
|
|
|
|
Table 4. Indications for Prescription Footwear and Insoles[55-58]
|
- Previous amputation
- Previous ulceration
- Preulcerative callus
- Peripheral neuropathy with evidence of callus formation
- Foot deformity
- Poor circulation
|
Table 5. Results of Comprehensive Program for Prevention of Lower Extremity Amputation
|
|
Diabetes Related Complications
|
Reduction After 1 Year of Comprehensive Foot Care
|
Emergency room visits
Hospitalization
Hospital days
Antibiotic prescription
Missed work (days)
Foot ulcer (days)
Foot operations
Lower extremity amputation |
81%
89%
90%
57%
70%
49%
87%
79% |
Sidebar: Keypoints
- Yearly foot examinations should assess skin, neurologic, vascular, and biomechanical status.
- Distal symmetric peripheral neuropathy affects 50% of diabetics 15 years after diagnosis, is best screened for by using a monofilament and may lead to muscle atrophy and deformity.
- Long, thick ingrown toenails and callus formation are risk factors for ulceration and should be trimmed.
- Prescription footwear and custom orthotics have been shown to prevent occurrence and recurrence of foot-related complications.
- Charcot (or neuropathic) fractures can be recognized by redness, swelling, warmth, bounding dorsalis pedis pulses, and absence or presence of pain. These fractures require immediate immobilization.
Send reprint requests to Bryan T. Green, MD, Duke University Medical Center, Division of Gastroenterology, Durham, NC 27710.
References
- Diabetes Facts: The Dangerous Toll of Diabetes. Alexandria, Va, American Diabetes Association, 1996
- Harris MI: Diabetes in America: epidemiology and scope of the problem. Diabetes Care 1998; 21(suppl 3):C11-C14
- Mokdad AH, Ford ES, Bowman BA, et al: Diabetes Trends in the US: 1990-1998. Diabetes Care 2000; 23:1278-1283
- American Diabetes Association: Clinical practice recommendations 2001. report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2001; 24(suppl 1):S12
- Rosenbloom AL, Joe JR, Young RS, et al: Emerging epidemic of type 2 diabetes in youth. Diabetes Care 1999; 22:345-354
- Pinhas-Hamiel O, Dolan LM, Daniels SR, et al: Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 1996; 128(5 pt 1):608-615
- Peters AL, Legorreta AP, Ossorio RC, et al: Quality of outpatient care provided to diabetic patients: a health maintenance organization experience. Diabetes Care 1996; 19:601-606
- Bailey TS, Yu HM, Rayfield EJ: Patterns of foot inspection in a diabetes clinic. Am J Med 1985; 78:371-374
- Hempel RJ: Physician documentation of diabetes care: use of a diabetes flow sheet and patient education clinic. South Med J 1990; 83:1426-1432
- Wylie-Rosett J, Walker EA, Shamoon H, et al: Assessment of documented foot examinations for patients with diabetes in inner-city primary care clinics. Arch Fam Med 1995; 4:46-50
- Cook CB, Penman A, Cobb AB, et al: Outpatient diabetes management of Medicare beneficiaries in four Mississippi fee-for-service primary care clinics. J Miss State Med Assoc 1999; 40:8-13
- American Diabetes Association: Position statement: preventive foot care in people with diabetes. Diabetes Care 2001; 24(suppl 1):S33-43,S56-S58
- American Diabetes Association: Economic consequences of diabetes mellitus in the US in 1997. Diabetes Care 1998; 21:296-309
- Hodgson T, Cohen A: Medical care expenditures for diabetes, its chronic complications and its comorbidities. Prev Med 1999; 29:173-186
- National Institute of Diabetes and Digestive and Kidney Diseases: Diabetic Neuropathy: The Nerve Damage of Diabetes. Washington, DC, US Department of Health and Human Services, 1995
- Mayfield JA, Reiber GE, Sanders LJ, et al: Preventive foot care in people with diabetes. Diabetes Care 1998; 21:2161-2177
- Cofield RH, Morrison MJ, Beabout JW: Diabetic neuroarthropathy in the foot: patient characteristics and patterns of radiographic change. Foot Ankle 1983; 4:15-22
- Armstrong DG, Todd WF, Lavery LA, et al: The natural history of acute Charcot's arthropathy in a diabetic foot specialty clinic. J Am Podiatr Med Assoc 1997; 87:272-278
- Reiber GE, Boyko EJ, Smith DG: Lower extremity foot ulcers and amputations in diabetes. Diabetes in America. Bethesda, Md, National Diabetes Data Group, National Institutes of Health, 2nd Ed, 1995, NIH Publication 409-429
- American Diabetes Association: Clinical practice recommendations. screening for type 2 diabetes. Diabetes Care 2001; 24:S20-S23
- Unwin N: Epidemiology of lower extremity amputation in centers in Europe, North America, and East Asia. Br J Surg 2000; 87:328-337
- Rith-Najarian S, Branchaud C, Beaulieu O, et al: Reducing lower extremity amputations due to diabetes: application of the staged diabetes management approach in a primary care setting. J Fam Pract 1998; 47:127-132
- Healthy People 2010: Objectives for Improving Health. Washington DC, US Department of Health and Human Services, Vol 1, 2nd Ed, 2000, pp 5-10
- Harrington C, Zagari MJ, Corea J, et al: A cost analysis of diabetic lower extremity ulcers. Diabetes Care 2000; 23:1333-1338
- Bloomgarden ZT: Nephropathy and neuropathy. American Diabetes Association Annual Meeting, 1999. Diabetes Care 2000; 23:549-556
- Edelson GW, Armstrong DG, Lavery LA, et al: The acutely infected diabetic foot is not adequately evaluated in an inpatient setting. Arch Intern Med 1996; 156:2373-2378
- Eckman MH, Greenfield S, Mackey WC, et al: Foot infections in diabetic patients: decision and cost-effective analysis. JAMA 1995; 273:712-720
- Apelqvist J, Ragnarson-Tennvall G, Persson U, et al: Diabetic foot ulcers in a multidisciplinary setting: an economic analysis of primary healing and healing with amputation. J Intern Med 1994; 23:463-471
- Gibbons GW, Marcaccio EJ, Burgess AM, et al: Improved quality of diabetic foot care, 1984 vs 1990: reduced length of stay and costs, insufficient reimbursement. Arch Surg 1993; 128:576-581
- Raviola CA, Nichter LS, Baker JD, et al: Cost of treating advanced leg ischemia. Arch Surg 1988; 123:495-496
- Mackey WC, McCullough JL, Conlon TP, et al: The costs of surgery for limb-threatening ischemia. Surgery 1986; 99:26-35
- National Institute of Diabetes and Digestive and Kidney Diseases: Feet Can Last a Lifetime: A Healthcare Provider's Guide to Preventing Diabetes Foot Problems. Washington, DC, US Department of Health and Human Services, 1997
- National Diabetes Data Group: Diabetes in America. Bethesda, Md, US Department of Health and Human Services, 1995
- Lavery LA, Ashry HR, van Houtom W, et al: Variation in the incidence and proportion of diabetes related amputation in minorities. Diabetes Care 1996; 19:48-52
- Centers for Disease Control and Prevention: Diabetes Fact Sheet: National Estimates and General Information on Diabetes in the United States. Atlanta, US Department of Health and Human Services, 1999
- Cutson TM, Bongiorni DR: Rehabilitation of the older lower limb amputee: a brief review. J Am Geriatr Soc 1996; 44:1388-1393
- Pernot HF, DeWitte LP, Lindeman E, et al: Daily functioning of the lower extremity amputee: an overview of the literature. Clin Rehabil 1997; 11:93-106
- Frykberg RG, Arora S, Pomposelli FB, et al: Functional outcome in the elderly following lower extremity amputation. JFoot Ankle Surg 1998; 37:181-185
- Walters RL, Perry J, Antonelli D, et al: Energy cost of walking of amputees: the influence of level of amputation. J Bone Joint Surg 1976; 58:42-46
- Pinzur MS, Gold J, Schwartz D, et al: Energy demands for walking in dysvascular amputees as related to the level of amputation. Orthopedics 1992; 15:1033-1037
- Lavery LA, Van Haitum WH, Armstrong DG: Institutionalization following diabetes-related lower extremity amputation. Am J Med 1997; 103:383-388
- Houghton AD, Taylor PR, Thurlow S, et al: Success rates for rehabilitation of vascular amputees: implications for preoperative assessment and amputation level. Br J Surg 1992; 79:753-755
- Coletta EM: Care of the elderly patient with lower extremity amputation. J Am Board Fam Prac 2000; 13:22-34
- Foster DW: Diabetes mellitus. Harrison's Principles of Internal Medicine. Fauci AS, Braunwald E, Isselbacher KJ, et al (eds). New York, McGraw-Hill Co, 14th Ed, 1998, pp 2060-2081
- Hotta N: New concepts and insights on pathogenesis and treatment of diabetic complications: polyol pathway and its inhibition. Nagoya J Med Sci 1997; 60:89-100
- Armstrong DG, Lavery LA, Vela SA, et al: Choosing a practical screening instrument to identify patients at risk for diabetic foot ulceration. Arch Intern Med 1998; 158:289-292
- Sinacore DR, Brown M, Carlson GS: Screening and assigning risk to the diabetic foot (Abstract). Issues Aging 1999; 22:25
- Magee DJ: Lower leg, ankle, and foot. Orthopedic Physical Assessment. Magee DJ (ed). Philadelphia, WB Saunders Co, 1997, pp 614-621
- Hampton GH, Birke J: Treatment of foot wounds caused by pressure and insensitivity. Wound Healing Alternatives in Management. McCulloch JM, Kloth LC, Feedar JA (eds). Philadelphia, FA Davis Co, 1995, pp 247-251
- Birke JA, Cornwall MW: Relationship between hallux limitus and ulceration of the great toe. J Orthop Sports Phys Ther 1988; 10:172-176
- Fromhertz WA: Examination. Physical Therapy of the Foot and Ankle. Hunt GC (ed). New York, Churchill-Livingstone, 1988, p 85
- Reiber GE, Vileikyte L, Boyko EJ, et al: Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999; 22:157-162
- Larsson J, Agardh CD, Apelquist J, et al: Long-term prognosis after healed amputation in patients with diabetes. Clin Orthop 1998; 350:149-158
- Kloth LC, McCulloch JM: The inflammatory response to wounding. Wound Healing Alternatives in Management. McCulloch JM, Kloth LC, Feedar JA (eds). Philadelphia, FA Davis Co, 2nd Ed, 1995, pp 13-14
- McGrath NM: Recent commencement of dialysis is a risk factor for lower-extremity amputation in a high risk diabetic population (Letter). Diabetes Care 2000; 23:432
- Eggers P, Gohdes D, Pugh J: Nontraumatic lower-extremity amputation in the Medicare endstage renal disease population. Kidney Int 1999; 56:1524-1533
- Lehto S, Ronnemaa T, Pyorala K: Risk factors predicting lower extremity amputations in patients with NIDDM. Diabetes Care 1996; 19:607-612
- Edmonds M, Foster AVM: Diabetic foot. Diabetic Complications. Shaw KM (ed). West Sussex, England, John Wiley and Sons Ltd, 1996, pp 150-156
- Murray HJ, Young MJ, Hollis S, et al: Association between callus formation, high pressure and neuropathy in diabetic foot ulceration. Diabetic Med 1996; 13:979-982
- Pitei DL, Foster A, Edmonds M: The effect of regular callus removal on foot pressures. J Foot Ankle 1999; 38:251-255
- Mueller MJ: Therapeutic footwear helps protect the diabetic foot. J Am Podiatr Med Assoc 1997; 87:360-364
- Janissee DJ: Prescription inserts and footwear. Clin Podiatr Med Surg 1995; 12:41-61
- Uccioli L, Faglia E, Monticone G, et al: Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care 1995; 18:1376-1378
- Kato H, Takada T, Kawamura T, et al: The reduction and redistribution of plantar pressures using foot orthoses in diabetic patients. Diabetes Res Clin Pract 1996; 31:115-118
- Wooldridge J, Moreno L: Evaluation of the costs to Medicare of covering therapeutic shoes for diabetic patients. Diabetes Care 1994; 17:541-547
- Pinzur MS: American orthopedic foot and ankle society diabetic shoe survey. Diabetes Care 1999; 22:2099-2100
- Wooldridge J, Bergeron J, Thornton C: Preventing diabetic foot disease: lessons from the Medicare therapeutic shoe demonstration. Am J Public Health 1996; 86:935-938
- Sugarman JR, Reiber GE, Baumgardner G, et al: Use of the therapeutic footwear benefit among diabetic Medicare beneficiaries in three states, 1995. Diabetes Care 1998; 21:777-781
- Schon LC, Easley ME, Weinfeld SB: Charcot neuroarthropathy of the foot and ankle. Clin Orthop 1998; 349:116-131
- Sanders LJ, Frykberg RG: Charcot foot. The Diabetic Foot. Levin ME, Oneil LW, Bouker JH (eds). St Louis, Mosley-Yearbook Inc, 5th Ed, 1993, pp 149-180
- Sinacore DR: Acute charcot arthropathy in patients with diabetes mellitus: healing times by foot location. J Diabetes Complications 1998; 12:287-293
- Fabrin J, Larsen K, Holstein PE: Long term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care 2000; 23:796-800
- Stevens MJ, Edmonds ME, Foster AVM, et al: Selective neuropathy and preserved vascular responses. the diabetic Charcot foot. Diabetolgia 1992; 35:148-154
- Sinacore DR, Withrington NC: Recognition and management of neuropathic (Charcot) arthropathies of the foot and ankle. J Orthop Sports Phys Ther 1999; 29:736-746
- Childs M, Armstrong DG, Edelson GW: Is Charcot arthropathy a late sequela of osteoporosis in patients with diabetes mellitus? J Foot Ankle Surg 1998; 37:437-439
- Lew DP, Waldvogel FA: Osteomyelitis. N Engl J Med 1997; 336:999-1006
- Jaakkola J, Kelh D: Hematogenous calcaneal osteomyelitis in children. J Pediatr Orthop 1999; 19:699-704
- Armstrong DG, Harkless LB: Outcomes of preventive care in a diabetic foot specialty clinic. J Foot Ankle Surg 1998; 37:460-466
- Mason J, O'Keeffee C, McIntosh A, et al: A systematic review of foot ulcer in patients with type 2 diabetes mellitus: prevention. Diabetic Med 1999; 16:801-812
- Patout CA Jr, Birke JA, Horswell R, et al: Effectiveness of a comprehensive diabetes lower-extremity amputation prevention (LEAP) program in a predominantly low income African American population. Diabetes Care 2000; 23:1339-1342
- Ollendorf DA, Kotsanos JG, Wishner WJ, et al: Potential economic benefits of lower-extremity amputation prevention strategies in diabetes. Diabetes Care 1998; 21:1240-1245
Melissa F. Green, PT, Zarrintaj Aliabadi, PhD, PA-C, Bryan T. Green, MD, Department of Internal Medicine and the Comprehensive Diabetic Foot Clinic, University of South Alabama, Mobile South Med J 95(1):95-101, 2002. © 2002 Southern Medical Association
|
|

