|
|
|||||||||||||||||||||||||||||||||||||||||
| Introduction
Diabetes and Retinopathy: the Scope of the Problem Most individuals with diabetes will ultimately develop diabetic retinopathy (DR). The likelihood of developing this complication depends on a variety of factors, including type and duration of diabetes, quality of glucose control, blood pressure, and serum lipid levels.[3-5] Duration of diabetes is the major variable related to the incidence of DR. The Centers for Disease Control and Prevention (CDC) reports that in the United States, up to 24,000 patients with diabetes become legally blind each year, and DR remains the leading cause of blindness in patients aged 20 to 74 years.[2] DR is also a major cause of blindness in the other industrialized countries of the world, and the increasing rates of diabetes incidence, coupled with a reduction in blindness due to other causes, will result in diabetic blindness becoming more statistically significant in other countries. Macular Edema The Importance of Screening and Treatment Early detection of significant DR remains the fundamental goal in the effort to reduce visual disability in patients with diabetes. Such screening efforts should be very cost-effective. One model[8] demonstrated a potential annual savings of $16.5 million for each additional 10% of the population with type 1 diabetes enrolled in screening and treatment. The Need for a Standardized Grading Scale In spite of the need for a more practical clinical grading system, no consensus has been reached on a common, practical, standard clinical terminology for the worldwide exchange of information and data. This has made it impossible for groups performing screening programs for DR and using customized grading schemes to compare their data with that obtained in other countries, where other severity grades were employed.[10] In several countries, simplified, condensed grading schemes have been developed in an effort to improve both communication among caregivers and screening of patients with diabetes.[11,12] Even though these schemes have improved communication among caregivers in their respective countries, none of these plans have gained worldwide acceptance, and there remains a genuine need for a single, practical disease-severity grading system that can be used around the world to facilitate communication across groups of practitioners. An optimal clinical grading system should be useful for a variety of caregivers with varying diagnostic equipment and skills, ranging from a retinal specialist with contemporary equipment to a trained physician assistant using only a direct ophthalmoscope. Implementation of care based on such a grading scheme would necessarily vary across different regions, because practice patterns and healthcare delivery systems for patients with diabetes mellitus differ around the world. A large consensus development workshop was held at the International Congress of Ophthalmology in Sydney, Australia, in April 2002. Even though participation was by invitation only, every attempt was made to involve interested parties from different parts of the world. Participants included retina specialists, comprehensive ophthalmologists, endocrinologists, and epidemiologists. See Appendix for a list of participants. Before the meeting, background materials synthesizing the need for and purpose of standard DR and DME disease severity classification systems, as well as a proposed initial scheme, were distributed to participants for the purpose of orientation. A modified nominal group technique, or modified Delphi technique,[16] was used to evaluate the level of consensus regarding this clinical classification, and participants were asked to describe their views on agreement via emailed questionnaires. (A 9-point rating scale was used, with "1" signifying strong disagreement and "9" signifying strong agreement. The results were mathematically aggregated to summarize the group results. To determine agreement and disagreement, a binomial distribution was applied. Depending on the number of participants, agreement was said to exist if there was a > 80% rate within a 3-point range of 1-3, 4-6, and 7-9. Disagreement was defined as >/= 20% rate in the 7-9 range, and at least another 20% in the 1-3 range. Otherwise, agreement was rated as "equivocal" or "partial," with many participants in the 4-6 range.) The initial statistical analysis of a pilot 4-stage rating system showed that there was relatively strong agreement regarding the pilot severity scale. There was no significant disagreement about the proposed classifications for either DR or DME severity. However, the range of responses was tighter for the DME classification. These group results were returned to all members of the group for comparison with their own individual ratings before participants were brought together at the workshop. The workshop in Sydney was organized to define the need, international scope, and scientific rationale of each item in the proposed classification of DR and DME, and then to address concerns and questions. A meeting facilitator structured the interactions by allowing each idea to be discussed in turn so that all participants would have the opportunity to express their perspectives at each step of the process. The most important proposed changes to the initial classification scheme included the addition of a level of "no apparent retinopathy" and a modification in the grading of DME. The workshop did not attempt to force consensus or create a final version by the end of the meeting. Rather, the revised classification, derived from the deliberations at the meeting, was developed and circulated to all workshop participants for comments and input for a period of 4-6 weeks before it was finalized. The responses of the workshop attendees were then re-evaluated in a fashion identical to that described above. On the basis of these results, there was strong agreement on what the final versions of the DR and DME clinical disease severity classifications should be; they are presented in Tables 1 and 2.
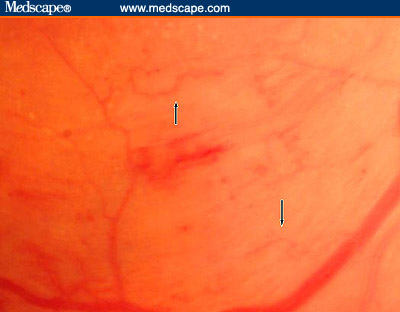
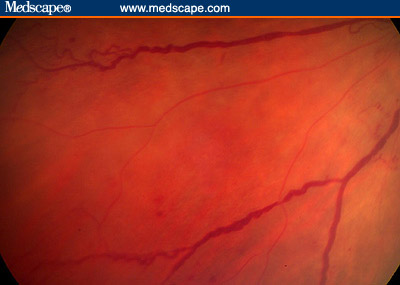
The DR Disease Severity Scale Most previously proposed grading scales recognized and recorded the following abnormalities: hemorrhages, microaneurysms, hard exudates, soft exudates ("cotton-wool patches"), intraretinal microvascular abnormalities (IRMA), venous beading (VB), new vessels < 1 disc diameter from the optic nerve (NVD), new vessels elsewhere (NVE), vitreous hemorrhages (VH), preretinal hemorrhages (PRH), and fibrous proliferations (FPD, FPE). In the proposed new classification, in addition to documenting the presence of some of the specific lesions mentioned above, the absence of diabetic retinopathy is documented as a distinct stage. The workshop participants believe that this is an important level, and, from both the patient's and physician's point of view, it represents a significant differentiation. Although an examiner might miss 1 or 2 microaneurysms, if a microaneurysm is definitely detected, this constitutes an important observation that can make a difference to a patient and to his or her primary care physician. Patients with no detectable signs of retinopathy may feel quite differently from patients with the beginning signs of retinopathy. Nevertheless, a finding of "microaneurysms only," the second stage in the proposed classification, is not associated with a significant risk of imminent proliferative disease or with a need for more than periodic reevaluation. Eyes with "no apparent DR" or "mild nonproliferative DR," as defined in the proposed disease severity system, were not included in the ETDRS. However, in the WESDR, the rate of progression to proliferative retinopathy 4 years after the initial evaluation was less than 1% for both young and older patients with diabetes and "no DR," whereas the risk in those with a "rare microaneurysm or hemorrhage" was 4.1 % in younger patients and considerably less in older patients.[13,14] The classification "moderate nonproliferative DR" in the proposed system includes eyes with ETDRS levels 35-47. The risk of progression to proliferative DR with high-risk features after 1 year was 1.2% for level 35, 3.6% for level 43, and 8.1% for level 47.[15] The fourth level of disease severity in the proposed classification "severe nonproliferative DR" indicates a major increase in risk. Continuing evaluations of ETDRS data have recognized the importance of IRMA (Figure 1) in the grading process, and a finding of severe IRMA in a single quadrant indicates the fourth level of severity. VB (Figure 2) is a second critical finding, and the presence of severe beading in 2 quadrants also indicates this fourth level. A large number (>/= 20) of hemorrhages in each of 4 quadrants is another finding that confirms the presence of "severe nonproliferative DR." The ETDRS "4:2:1 rule" indicates that the presence of severe hemorrhages in 4 quadrants, or VB in 2 quadrants, or IRMA in a single quadrant represents this severity of retinopathy, and the workshop panel agreed that the 4:2:1 rule should remain the basis of classifying an eye as having reached this level. ETDRS data indicate that, in eyes at this stage, the rate of developing proliferative DR with high-risk characteristics within 1 year is approximately 17%, and within 3 years it is more than 40%.[15] Figure 1. Severe IRMA (arrows) and intraretinal hemorrhage. Figure 2. Severe venous beading. It is difficult to recognize IRMA and VB. Thus, surrogate markers such as hemorrhages and/or microaneurysms (H/MA) have been evaluated. In preparation for the development of this classification system, Ronald Klein, MD, (personal communication, 2002) re-evaluated data from the WESDR for the association of specific lesions with the severity of DR. The use of H/MA alone to predict the risk of proliferative DR was demonstrated to be inadequate, because there was a lack of concordance of these lesions with the presence of IRMA and VB. For right eyes with IRMA present, 41% of patients with type 1 diabetes and 38% of patients with type 2 diabetes did not have H/MA >/= ETDRS standard photograph #1 in 1 or more fields. For right eyes with VB present, 29% of patients with type 1 diabetes and 15% with type 2 diabetes did not have H/MA >/= standard photograph #1 in 1 or more fields. The sensitivity of using H/MA >/= ETDRS standard photograph #1 in 1 or more fields for detecting the presence of IRMA or VB is about 60% to 64%, and the specificity is about 85% to 94%. Similarly, the presence of hard exudates or soft exudates was not very predictive of IRMA and/or VB. Therefore, it is necessary to identify the specific lesions as IRMA and/or VB -- and not rely on H/MA or exudates alone -- to differentiate the third level ("moderate") from the fourth level ("severe") of nonproliferative DR. It is important that all examiners appreciate this reality, and ETDRS photographs of IRMA and VB are included with a description of the grading scheme. Eyes with findings less severe than the 4:2:1 rule but more than "microaneurysms only" are classified in the third stage, "moderate nonproliferative DR." The presence of apparent neovascularization in any location was the basis for grading an eye as being at the fifth stage in the grading classification, "proliferative DR." No subdivisions of neovascularization, based on location or size, were considered critical. All ETDRS categories of proliferative DR were collapsed into this single fifth level of disease severity.
The initial grading decision involves a determination of the presence of any retinal thickening in the posterior pole. If the examiner considers him- or herself capable of further defining retinal thickening in terms of its extent, 3 grades of edema severity are differentiated. These are related to the distance of the thickening from the center of the retina. The distinction between "distant" and "approaching" is admittedly vague, but it is especially important to differentiate eyes that have no thickening of the central macula from those that do. Implementation of this system will rely on its dissemination to all physicians and others involved in the treatment of patients with diabetes. Training regarding the recognition of important lesions will be required in many locales. Ideally there will be a process to test this system in a variety of local settings and to re-evaluate its feasibility and utility in busy clinical environments. Different localities and systems of care will vary in their approaches to implementation and will employ different care providers and care delivery processes to manage patients with diabetes. Individualized treatment plans will also vary according to individual clinical considerations. Many of these are not included in this classification but have an important role in the risk of disease progression and in managing individual patients. Still, the most important steps that must be taken to reduce the rate of vision loss due to DR are to increase the percentage of diabetes patients that are screened and to improve the ability of clinicians to detect significant DR. This proposed disease severity classification should enhance abilities to accomplish the second step. Current treatment strategies for appropriate stages of DR are quite effective in reducing the odds of blindness, and future methods of treatment may prove to be even more beneficial. Author: Charles P. Wilkinson, MD (for the Global Diabetic Retinopathy Project Writing Team) References
Abstracts available on https://profreg.medscape.com |
|||||||||||||||||||||||||||||||||||||||||