|
|
|||
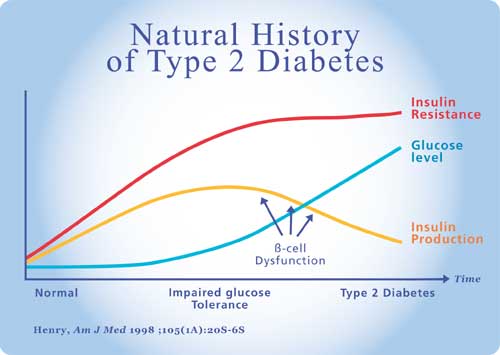
| Type 2 Diabetes: the problem Type 2 Diabetes Mellitus is a metabolic disease characterized by hyperglycemia due to defective insulin secretion, insulin action or both. It is the defective insulin action or Insulin Resistance that is one of the greatest challenges in Diabetes management. Traditionally our thinking has been that it is the chronic glucose elevation of diabetes that leads to the damage and dysfunction to the kidney, eye, nerves and blood vessels. We are now realizing that the risks and damage may start years before blood glucose levels rise above normal. There is currently an epidemic of Type 2 Diabetes throughout the world that is rapidly worsening, the number of cases in Canada is expected to double between 2000 and 2010. The cost in lives lost and the financial cost of dealing with the medical complications of diabetes is staggering. It is only by understanding and developing effective treatment for Insulin Resistance that we can hope to deal to this threat to our lives and health. Pathogenesis of Type 2 Diabetes Insulin resistance is defined as an impaired biologic response to either exogenous or endogenous insulin (12). Insulin resistance by itself does not cause diabetes. As long as the pancreatic beta cell can compensate for the insulin resistance by producing more insulin; glucose levels will remain normal. It is only when the beta cell becomes impaired and insulin secretion is inadequate to compensate for insulin resistance that glucose levels rise. Initially there may be adequate insulin production in the fasting state but an inability for the pancreas to cope with the stress of high carbohydrate intake resulting in post prandial hyperglycemia. This stage may be diagnosed by an elevated 2 hr glucose on the standard glucose tolerance test that is above 7.8 mmol/L but below the diagnostic value of 11.1 mmol/L for diabetes mellitus. This stage is known as impaired glucose tolerance and is a precursor of Type 2 Diabetes.
Initially insulin resistance is compensated by hyperinsulinism; as the beta cell becomes exhausted and can no longer keep up, we develop impaired glucose tolerance (IGT). As the insulin secretory defect progresses, glucose levels rise still further and when the fasting plasma glucose level exceeds 7 mmol/L or the after meal glucose level exceeds 11.1 mmol/l, we say that diabetes has been diagnosed. The diagnosis of Diabetes is based on a glucose level but the disease that caused this glucose level has been present for years. The first manifestation of disease has been insulin resistance and elevated serum insulin levels. In 1988 Gerald Reaven recognized a cluster of risk factors commonly present in individuals with high insulin levels (Reaven G. Role of insulin resistance in human disease. Diabetes 1988; 37: 1595-1607). This was initially referred to as syndrome X and is characterized by hypertension, obesity (particularly abdominal), high triglyceride, low HDL and impaired glucose tolerance. Insulin resistance is the common denominator of the syndrome. In 1998 the World Health Organization called this The Metabolic Syndrome. Individuals with the metabolic (insulin resistance) syndrome are at dramatically elevated risk for diabetes, ischaemic heart disease, stroke, kidney failure, blindness and nerve disease. People with diabetes have up to four times the risk of developing ischaemic heart disease of age matched non diabetics. What Happens in Insulin Resistance Normal Metabolism Insulin stimulates production of triglycerides from free fatty acids and glycerol. In the fasting state when insulin levels are low, triglycerides are broken down by lipolysis to free fatty acids and glycerol. The catecholamine stress hormones also break down triglycerides. Free fatty acids can be oxidized in the mitochondria to provide energy. Protein metabolism consists of breakdown of protein to amino acids and synthesis of protein from amino acids. In times of need, glucose can be synthesized from amino acids. Insulin inhibits protein breakdown and stimulates protein synthesis while glucagon and low insulin levels favour protein breakdown. Metabolic Disturbances in Insulin Resistance In the normal individual, hepatic glucose production is suppressed by insulin. In susceptible individuals there is impaired suppression of hepatic glucose production by insulin. In the early stages, the decreased glucose disposal (from decreased glycogen formation) and the increased glucose production (by the liver) are compensated by increased insulin production by the pancreas so glucose levels remain normal. As the disease progresses, the pancreatic beta cell production decreases and and is unable to keep up with the body's needs in times of stress. The production of insulin cannot keep pace with acute needs and and initially early phase insulin secretion is lost. The loss of first phase insulin secretion leads to post prandial hyperglycemia. As the condition progresses with time, post prandial glucose levels increase, when these levels reach the level of 9 mmol/L we say that the individual has impaired glucose tolerance, and when the post prandial level exceeds 11.1 mmol/L we diagnose Diabetes Mellitus but these levels are mere points on a continuum of dysfunction that first started years or decades earlier with decreased glycogen production leading to decreased glucose disposal. The transition from normal glucose tolerance to IGT and to Type 2 Diabetes is a reflection of the deterioration of the function of the pancreatic beta cell (2). What causes insulin resistance? Insulin resistance can be triggered by obesity, pregnancy, aging or infections. Obesity and particularly abdominal obesity is associated with decreased levels of insulin mediated glucose uptake but is the obesity the cause or the effect of insulin resistance (3). Abdominal fat tissue could provide a chain of events leading to skeletal muscle insulin resistance which appears to be the first step in the cascade leading ultimately to Type 2 Diabetes. There are certainly genetic factors in the development of Type 2 Diabetes and the first of these may be the genetic factor for abdominal obesity (4). Another factor is possibly the “Thrifty Gene”.
We know that the risk of microvascular disease (retinopathy, nephropathy, neuropathy) increases directly with glucose levels and this is one reason why the diagnostic levels of glycemia were changed in the 1998 CDA guidelines for diagnosis of Diabetes. The old fasting glucose level for diagnosis of diabetes had been 7.8 but at this level 20% of newly diagnosed diabetics already had microvascular disease. What tends to be less well known is that the threshold of glycemia for development of macrovascular disease is much lower. The Honolulu heart study showed that the risk of ischaemic heart disease was elevated at post prandial glucose levels of above 5.2 mmol/L . We have defined glycemic levels for the diagnosis of diabetes on the basis of microvascular disease risk, if instead we had used the risk for macrovascular disease our dignostic levels would be much lower, probably a fasting glucose of about 4 mmol/L and a post prandial level of 5 mmol/L. Most people with insulin resistance already have elevated glucose levels though they may not yet be in the diabetic range, this increased level of basal glycemia increased the risk for ischaemic heart disease. The major cause of death in type 2 diabetics and in people with impaired glucose tolerance is ischaemic heart disease. The cardiac risk of type 2 diabetes is the same as having had a previous coronary event (7). Individuals with insulin resistance and type 2 diabetes have abnormal lipids including elevations of triglycerides and low HDL (8). In the United Kingdom Prospective Diabetes Study (UKPDS) (9), men with diabetes had elevated triglyceride levels and lower HDL compared to control while women had the same elevated triglyceride values and low HDL but they also showed higher LDL than controls. The composition of the HDL and LDL particles is also different in subjects with insulin resistance, IGT and type 2 diabetes with a decrease in particle size of both HDL and LDL. The decreased particle size of the HDL confers less protection against heart disease while the smaller denser LDL particles are more easily oxidized and are more atherogenic (10). In insulin resistance and type 2 diabetes there is enhanced clotting and inhibited clot breakdown which explains the increased risk of acute coronary occlusion and myocardial infarction. There are increased levels of fibrinogen, plasma activator inhibitor-1 (PAI-1), factor V and D-dimer; all of which contribute to enhanced thrombogenesis as well as decreased fibrinolysis (11). Platelet abnormalities are also seen with an increased sensitivity to aggregation. The platelets are more sensitive to aggregating agents such as epinephrine, thromboxane and thrombin as well as having increased glycoprotein receptors (11). Insulin resistance contributes to endothelial dysfunction by stimulating smooth muscle cell proliferation, stimulating growth factors, increasing formation and decreasing regression of lipid plaques and by stimulating connective tissue synthesis. Insulin resistance contributes to insulin induced hypertension by enhancing renal tubular reabsorption of sodium and increasing the tone of the sympathetic nervous system. The result of these lipid, glucose and hemostatic abnormalities results in increased risk of coronary heart disease and worsens the prognosis following a coronary event. 50% of type 2 diabetics will die from coronary ischaemic events and of those that suffer an MI, 44% will be dead in the next year. Treatment of insulin resistance is therefore of paramount importance in decreasing morbidity and mortality. Insulin resistance is the first abnormality seen in the individual who will develop type 2 diabetes. Initially there is hyperinsulinism but as the pancreatic beta cell is no longer able to produce the increased amounts of insulin needed for glucose control; relative insulin deficiency results and glucose levels start to rise. When a critical plasma glucose level is reached, we diagnose diabetes. The hyperinsulinism and the cluster of related symptoms such as hyperlipidemia, obesity, hypertension, hypercoagulability and microalbuminuria lead to increased risk of death and illness. Logically we should direct our treatment to the reduction of insulin resistance. Lifestyle Measures Exercise: Exercise is one of the most effective means at our disposal to increase non-insulin dependant glucose transport. Exercise is therefore one of the cornerstones of treatment of type 2 diabetes. Diet: Even a modest weight loss of 5% of total body weight can lead to a significant improvement in insulin resistance and glycemic control as well as improving lipid profile and lowering blood pressure. Pharmacologic treatment: The object of pharmacologic treatment should be to improve insulin resistance and to reduce glucose levels. Classes of Oral Antidiabetic Medications:
The enhanced glucose transport leads to decreased glucose levels and increased glycogen formation. It is known that elevated plasma free fatty acids which are seen in insulin resistance and type 2 diabetes impair glucose transport. The glitazones impair breakdown of triglyceride leading to lowering of plasma free fatty acids and therby improving glucose transport. Increased free fatty acids also lead to increased liver gluconeogenesis and decreased glycolysis so the decrease in FFA decreases gluconeogenesis, increases glycolysis and lowers plasma glucose. By decreasing hepatic phosphenolpyruvate carboxykinase (PEPCK) the glitazones reduce hepatic insulin resistance. References
|
|||